The Ten Principles Behind Arterial Pressure

Highlights
- Arterial pressure is the result of the interactions between ventricular contraction, the ventricular hydraulic load and the arterial system [1]. Extravascular mechanical forces, such as intra-thoracic and intra-abdominal pressures, acting on the vessels further affect arterial pressure (View Highlight)
- The major determinants of P sys are the pattern and duration of ventricular ejection (stroke volume), the distensibility (compliance) of the arterial vessels, the velocity of the pressure wave in large arteries and the degree of pressure wave reflection in peripheral arteries, the latter being influenced by vasomotor tone [1, 2]. An increase in transmission velocity of both the forward and reflected pressure waves leads to arrival of the reflected wave in the central aorta during systole, thus augmenting pressure (View Highlight)
- The P dias depends on arterial compliance, which decreases as arterial stiffness increases, as well as on heart rate and the resistance related to the length and diameter of vessels and the distribution of the vascular network (series and parallel circuits). (View Highlight)
- As the aortic pressure pulse travels away from the aorta to the distributing arteries, P sys rises and P dias falls as a result of reflected waves in the branching vessels and the decreased arterial compliance of the distributing arteries. Consequently, the pulse pressure increases from the aorta to the systemic arteries (View Highlight)
- Increased stiffness of the central elastic arteries is the major cause of augmented systolic and pulse pressure in aged and hypertensive patients [1, 2]. The amplitude of pulsation progressively decreases in the smaller arteries and arterioles and is minimal in the capillaries because of increased resistance and reduced compliance in smaller vessels (View Highlight)
- The mean aortic pressure (MAP) is the average pressure value during the aortic pulse cycle. The relatively low resistance in the aorta and in the distributing arteries means that there is only a small decrease in MAP as the aortic pressure pulse travels away from the aorta to small arteries [1]. MAP can be therefore be used as a reference value along the arterial system to estimate arterial pressure. (View Highlight)
- The sympathetic nervous system and baroreflex functions play pivotal roles in coupling the left ventricle to the arterial circulation. Sympathetic modulation of afterload allows the left ventricle to generate stroke volume at the lowest energy expenditure (View Highlight)
- Ventricular afterload can be defined as the hydraulic input impedance presented to the ventricle and can be expressed as ascending aortic impedance [1, 3,4,5] that includes both static (total peripheral resistance) and pulsatile (stiffness and reflection) components (View Highlight)
- Ideally, central aortic pressure can be considered to be a surrogate of ventricular wall tension and is thus the closest estimate of afterload (View Highlight)
- Changes in vascular tone, for example those related to sepsis, affect both the amplitude and timing of the reflected waves so that it is difficult to relate peripheral to central pressures in critically ill patients (View Highlight)
- Intermittent positive-pressure ventilation induces cyclic changes in stroke volume, which in turn leads to cyclic changes in pulse pressure, with increases during inspiration and decreases during expiration. Such pulse pressure variations may be particularly pronounced in preload-dependent conditions and may thus be used to predict the hemodynamic response to a fluid challenge (View Highlight)
- neither changes in pulse pressure nor changes in arterial pressure reflect changes in cardiac output during a fluid challenge (View Highlight)
- Modulation of local vascular resistance at the microcirculatory level is the main determinant for blood flow distribution according to metabolic needs (View Highlight)
- Capillary perfusion depends on the difference between inflow pressure and outflow pressure, and vasodilation is the main mechanism for increasing local blood flow. Hence, a sufficient inflow pressure must be maintained to preserve organ perfusion (View Highlight)
- The inflow pressure is regulated by the vasomotor tone of arterioles and pre-capillary sphincters along the vascular system and depends on the pressure difference between the central arterial pressure (i.e. MAP) and arterial critical closing pressure (P cc, the pressure threshold that coincides with the stop of organ blood flow) (View Highlight)
- The outflow pressure is the pressure difference between the mean systemic filling pressure (P msf) and the right atrial pressure (RAP) (View Highlight)
- The arterial and venous resistances determine the “vascular waterfall” that contributes to the maintenance of organ perfusion even in low-flow conditions [8,9,10,11]. Even if MAP decreases to P cc, a pressure gradient between P cc and P msf is still maintained (View Highlight)
- The presence of the vascular waterfall explains why changes in RAP minimally affect capillary flow (View Highlight)
- Inflow pressure thresholds (MAP − P cc) and outflow pressure vary between organs and may be affected by interstitial pressure (View Highlight)
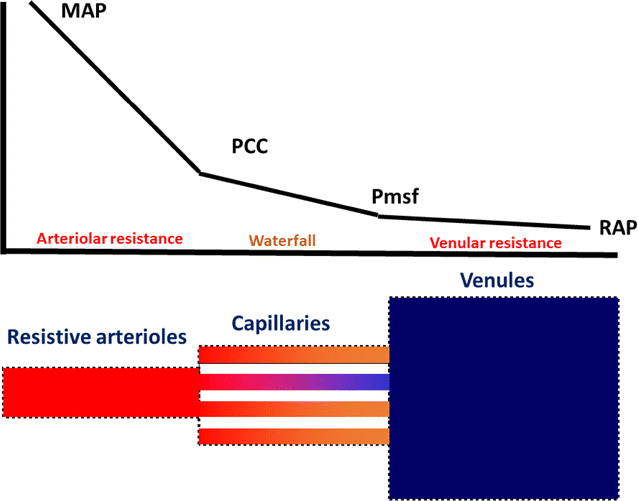
Schematic representation of the pressure drop along the vascular system. Graphic representation of the pressure drop from the mean arterial pressure (MAP) to the critical closing pressure (P cc ), and from the mean systemic filling pressure (P msf ) to the right atrial pressure (RAP). The pressure gradient between P cc and P msf constitutes the “vascular waterfall”. The precise location of P msf is not well defined. The presence of a vascular waterfall indicates there are two separate but sequential vascular resistances (arteriolar resistance and venular resistance) which determine the pressure drop, rather than a unique total systemic vascular resistance (View Highlight)- In contrast to acute low flow states in which MAP decreases and P cc remains unaffected, the massive loss of smooth muscle tone in sepsis decreases both MAP and P cc. The P cc may thus become equal to the downstream pressure (P msf), rendering the vascular waterfall ineffective (View Highlight)
- When MAP is below the autoregulation threshold and the vascular waterfall is eliminated, tissue perfusion becomes solely dependent on perfusion pressure and thus MAP (View Highlight)
- If the vascular waterfall is absent, an increase in RAP may further worsen capillary perfusion (View Highlight)
- Vasopressor agents increase MAP but the effect on P cc remains to be better elucidated (View Highlight)
- As the arterial waveform is determined by both incident and reflected waves, different arteries (radial, brachial, femoral) produce different waveforms within the same individual. (View Highlight)
- The treatment goal for vasopressors in hypotension is to increase MAP above the autoregulatory threshold to preserve tissue perfusion (View Highlight)

